Abstract
Introduction: L-glutamine (Endari ®, Emmaus Medical, Inc.) is an amino acid approved for the treatment of sickle cell disease (SCD) by the United States Food and Drug Association (FDA) after 2 clinical trials demonstrated a reduction in painful episodes and hospitalizations. L-glutamine has many roles, including de novo synthesis of intracellular glutathione that may protect sickle erythrocytes from oxidant injury. Despite its licensure, the pharmacokinetics (PK) of multi-dose oral L-glutamine for SCD have not been studied.
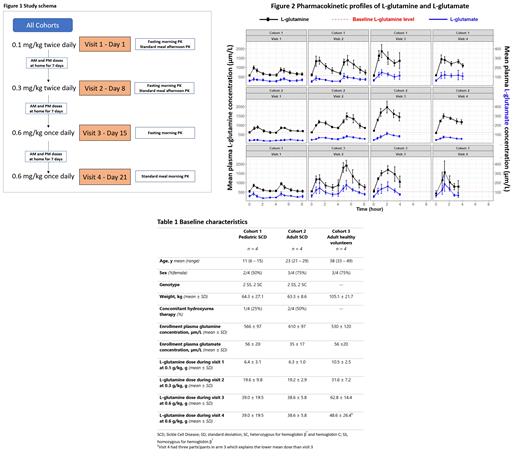
Methods: We conducted a dose-escalation PK trial from January to June 2021 (NCT04684381) on 3 cohorts with 4 participants each: pediatric SCD patients (0 - 18 y, cohort 1), adult SCD patients (>18 y, cohort 2), and adult healthy volunteers (>18 y, cohort 3). There were 4 weekly study visits to assess 3 ascending dose levels (Figure 1). For each visit, participants arrived in the research clinic after an overnight fast. Plasma L-glutamine and other amino acid levels were obtained before and after oral L-glutamine doses at each visit and quantified by ion exchange chromatography.
On study visit 1, a dose of 0.1 g/kg of L-glutamine was administered and amino acids were measured at 30±5, 60±10, 120±15, 180±15 and 240±20 minutes after the dose. The procedure was repeated in the afternoon after a standardized meal. On study visit 2, the dose was increased to 0.3 g/kg twice daily (approximate FDA-approved dosing). On study visit 3, a single oral dose of 0.6 g/kg (once daily dosing) was given for fasting PK levels, and on study visit 4 the 0.6 g/kg dose was given with a standardized meal. After the first 3 study visits, participants continued L-glutamine at the corresponding dose level at home and completed a daily phone survey to document adherence and adverse events.
Results: Baseline characteristics are summarized in Table 1. Baseline L-glutamine levels similar across the three study cohorts (Table 1). One participant from cohort 1 withdrew during study visit 1 and was replaced. Another participant from cohort 2 was withdrawn due to hospitalization for severe anemia and was replaced. One participant from cohort 3 did not complete visit 4 due to dyspepsia.
Non-compartmental PK analysis (NCA) was conducted using Phoenix WinNonlin® Professional Version 8.4 (Certara, NJ, US; Figure 2). After oral administration, L-glutamine plasma levels peaked (T max) at an average (mean ± SD) of 1.2 ± 0.9 hours. Compared to doses 0.1 g/kg and 0.3 g/kg, the T max was later for 0.6 g/kg (p<0.05) indicating slower absorption at higher doses. At the lowest dose (0.1 g/kg), L-glutamine concentrations returned to baseline levels within 4 hours. However, at 0.3 or 0.6 g/kg, the plasma L-glutamine concentration remained higher than baseline at 4 hours, indicating slower elimination at higher doses. There was no significant change in the baseline plasma L-glutamine concentration levels during the study, however, indicating no evidence of drug accumulation. Peak L-glutamine concentration levels (C max) were higher at each successive dose escalation, but when the levels were normalized by dose there was a decrease in the normalized concentration with dose escalation, which implied a potential saturable absorption. Inter-patient variability in plasma L-glutamine levels was higher at 0.6 mg/kg than at lower doses. The elimination half-life (T 1/2), calculated as 0.693/elimination rate constant (λz), had an average value of 1.0 ± 0.7 hours.
The most common grade 2 adverse events were dyspepsia and headache (3 instances each). There was no significant elevation in ammonia levels, and plasma concentrations of the metabolite L-glutamate was similar to L-glutamine (Figure 2).
Conclusions: This is the first multi-dose PK study of L-glutamine (Endari) for SCD. Daily dosing was overall well-tolerated, with rapid absorption of 0.1 and 0.3 g/kg doses and no drug accumulation. The highest 0.6 g/kg dose was limited by palatability and featured slower and saturable absorption, so further dose escalation is likely not feasible. Ongoing analyses include possible food-drug interactions and PK-pharmacodynamic (PK-PD) studies to identify salutary effects on erythrocytes. Our findings will help inform the proper dosing of L-glutamine for patients with SCD, and potentially permit individualized PK-guided dosing to maximize treatment benefits.
This was an investigator-initiated trial funded by Emmaus Medical, Inc.
Sadaf: Emmaus Medical, Inc: Research Funding. Ware: Bristol Myers Squibb: Research Funding; Addmedica: Research Funding; Hemex Health: Research Funding; Nova Laboratories: Research Funding; Novartis: Other: DSMB Chair; Editas: Other: DSMB Chair. Quinn: Emmaus Medical: Research Funding; Novo Nordisk: Consultancy; Aruvant: Research Funding; Forma Therapeutics: Consultancy.
Endari (L-glutamine) is FDA approved for the treatment of Sickle Cell Disease at a dose of 0.3 g/kg. However, in our pharmacokinetic study we tested doses 0.1, 0.3 and 0.6 g/kg.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal